22 recall the positions of metals and nonmetals in the Periodic Table From left to right across a period there is a gradual change from metal to nonmetal elements For example, in Period 3, sodium, magnesium and aluminium are metals They allThe periodic table Mendeleev made an early periodic table In the modern periodic table, elements are in order of atomic number in periods andThis slideshow covers the new AQA 16 GCSE content on Chemistry unit 1, including the structure of the atom, elements, compounds, balancing equations, naming compounds, mixtures, filtration, evaporation, distillation, chromatography, the development of atomic structure theory, Rutherford scattering, mass and atomic number, isotopes, electron structure, the periodic table, how the periodic table

History Of The Periodic Table Gcse Chemistry Combined Science Aqa Revision Study Rocket
Periodic table of elements gcse chemistry
Periodic table of elements gcse chemistry-GCSE Chemistry (Single Science) The periodic table and properties of elements learning resources for adults, children, parents and teachersA printable periodic table of the chemical elements displaying the atomic number, symbol, name and atomic weight



Pass My Exams Easy Exam Revision Notes For Gsce Chemistry
Periodic Table of the Elements by revisioncentre 19 April 19 April GCSE Chemistry (Elements highlighted in a red font are important elements which you should really know about) Search for Shop Online Recent Posts Resilience Lessons from the School ofThe periodic table is classified so that elements are arranged in order of increasing atomic number across a grid >Gcse Atoms Elements Showing top 8 worksheets in the category Gcse Atoms Elements Some of the worksheets displayed are Gcse grade, Ks3 chemistry elementsatoms, Topic 1 atomic structure and the periodic table, Aqa ocr edexcel gcse science, Aqa gcse 9 1 chemistry, Compounds and mixtures, Gcse chemistry revision notes 21, Aqa ocr edexcel gcse science
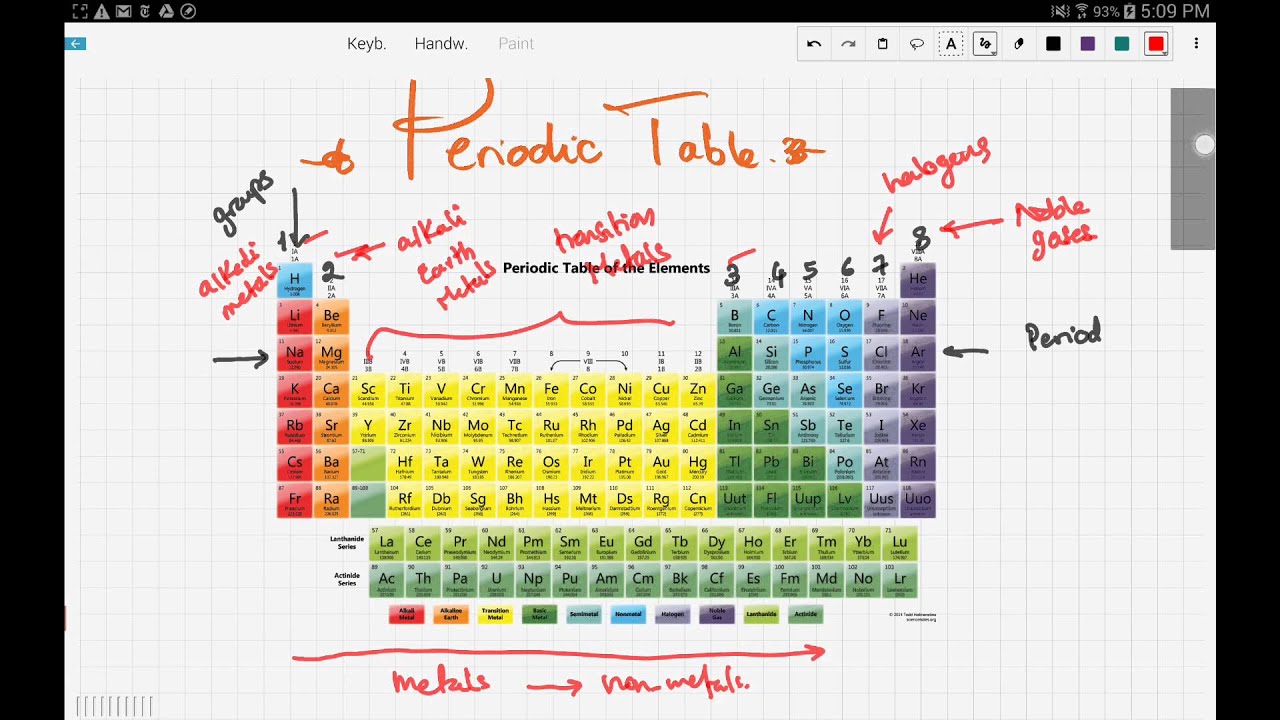
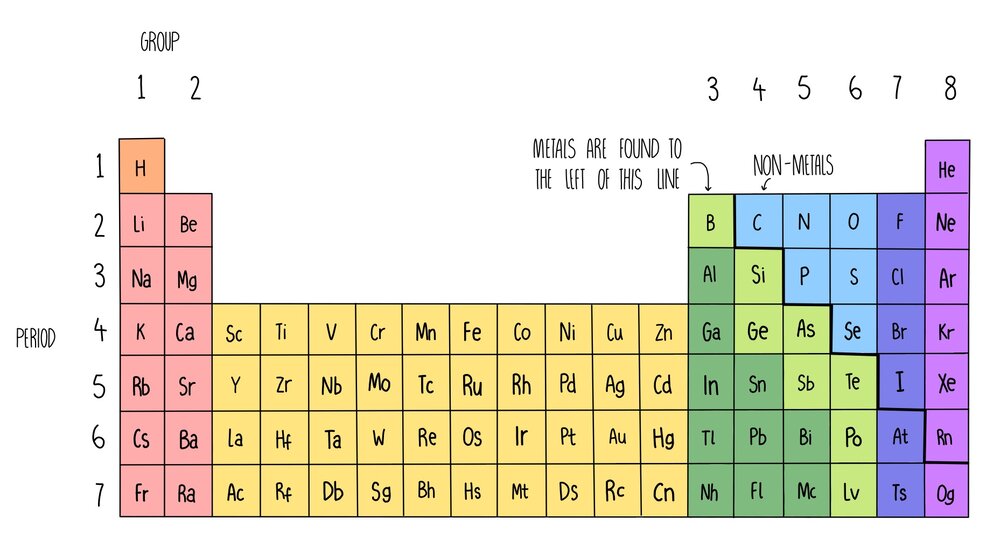
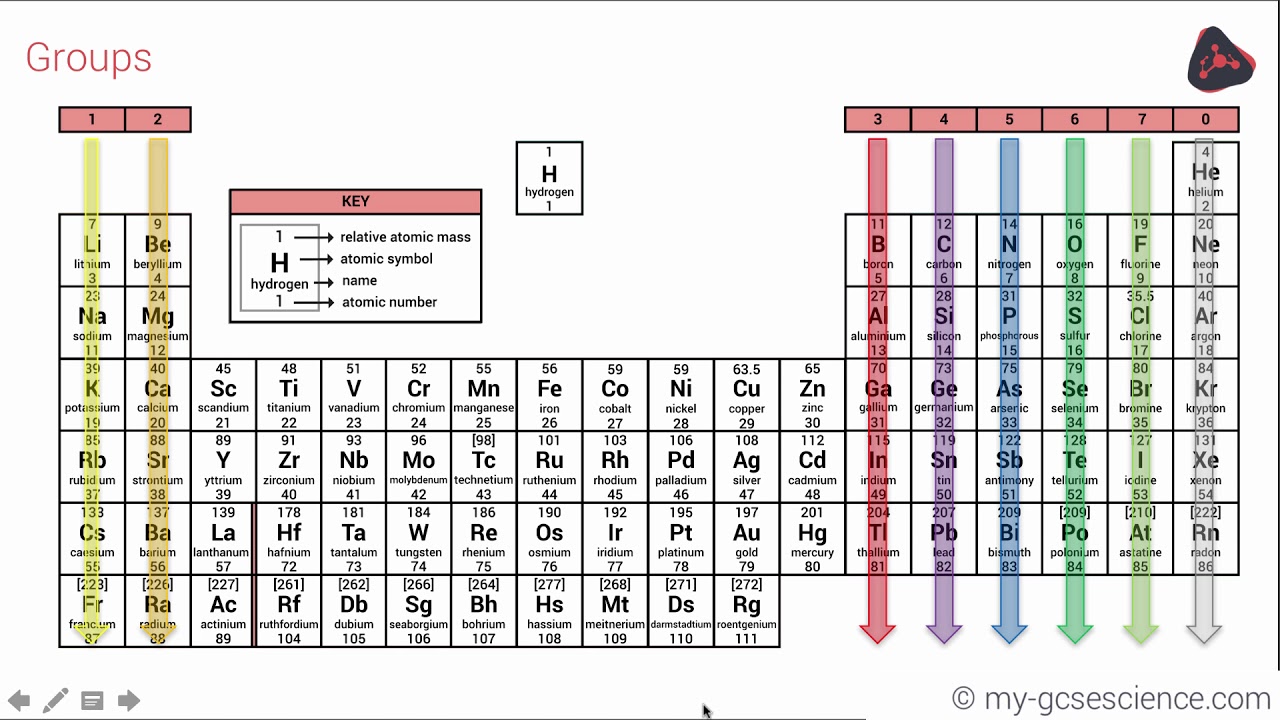
The Periodic Table This modern periodic table lists elements according to their atomic number, if they were arranged according to atomic mass potassium and argon would be the wrong way round Elements having the same number of electrons in their outermost shell are placed in vertical columns called groups They have similar chemical propertiesPearson's Edexcel International GCSE Chemistry books The Periodic Table Online Periodic Tables WebElements Masses of useful information about the elements and their simple compounds The only downside is its obtrusive advertisements Fast Periodic Table From wwwschoolsciencecouk (produced by the UK Association for Science Education)There are more than 100 different elements The periodic table is a chart showing all the elements arranged in order of increasing atomic number The vertical columns in the periodic table are
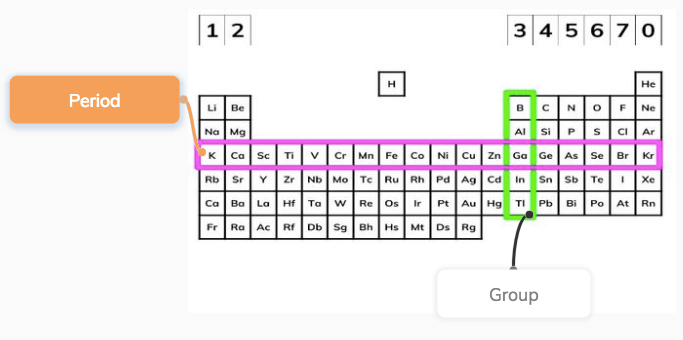
Elements are arranged on the Periodic Table in order of increasing atomic number, where each element has one proton more than the element preceding it The table is arranged in vertical columns called Groups numbered 1 – 8 and in rows called Periods Period these are the horizontal rows that show the number of shells of electrons an atom hasSilicon 14 2,8,4 Phosphorus 15 2,8,5 Sulfur 16 2,8,6 Chlorine 17 2,8,7 Argon 18 2,8,8 Potassium 19 2,8,8,1 Calcium 2,8,8,2 Note although the third shell can hold up to 18 electrons, the filling of the shells follows a more complicated pattern after potassium and calcium For these two elements, the third shell holds 8 and theFor Students Your Profile;
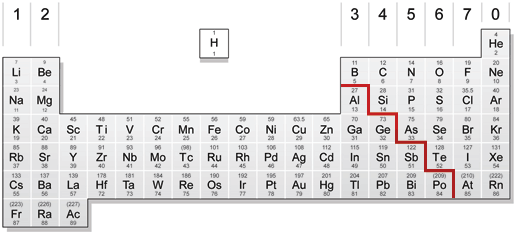



The Periodic Table Revision Notes Igcse Chemistry Oxnotes Gcse Revision



Periodic Table Elements Flashcards
Learn who made the periodic table, why he arranged the elements this way and about the important groups you need to knowChemistry The Periodic Table (AQA) A basic understanding of the fundamental ideas in chemistry is required of students in GCSE Science This is the second of six quizzes going over these fundamental ideas and it looks specifically at the periodic table The periodic table is a fundamental part of chemistry and we take it and its usefulness for granted, but that hasn't always been theThe periodic table below is based on the ones used by the different examination boards The group numbers 1 to 0 (the top ones) are used in most GCSE courses The group numbers 1 to 18 were recommended by IUPAC in 19 At the moment these are only used in OCR courses There is a summary at the bottom of the page It shows the differences
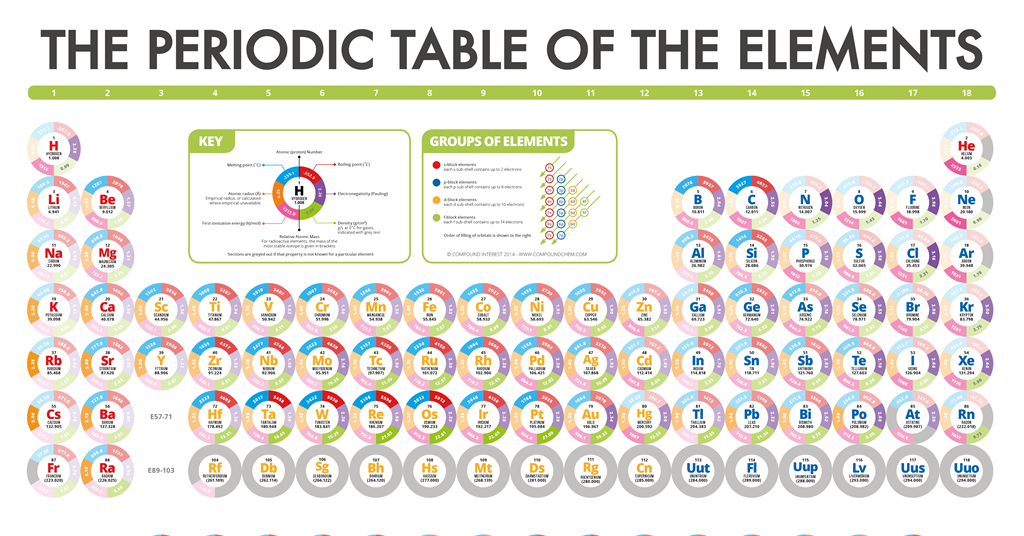



Elements Infographics Resource Rsc Education



Gcse Chemistry Introductory Unit Page
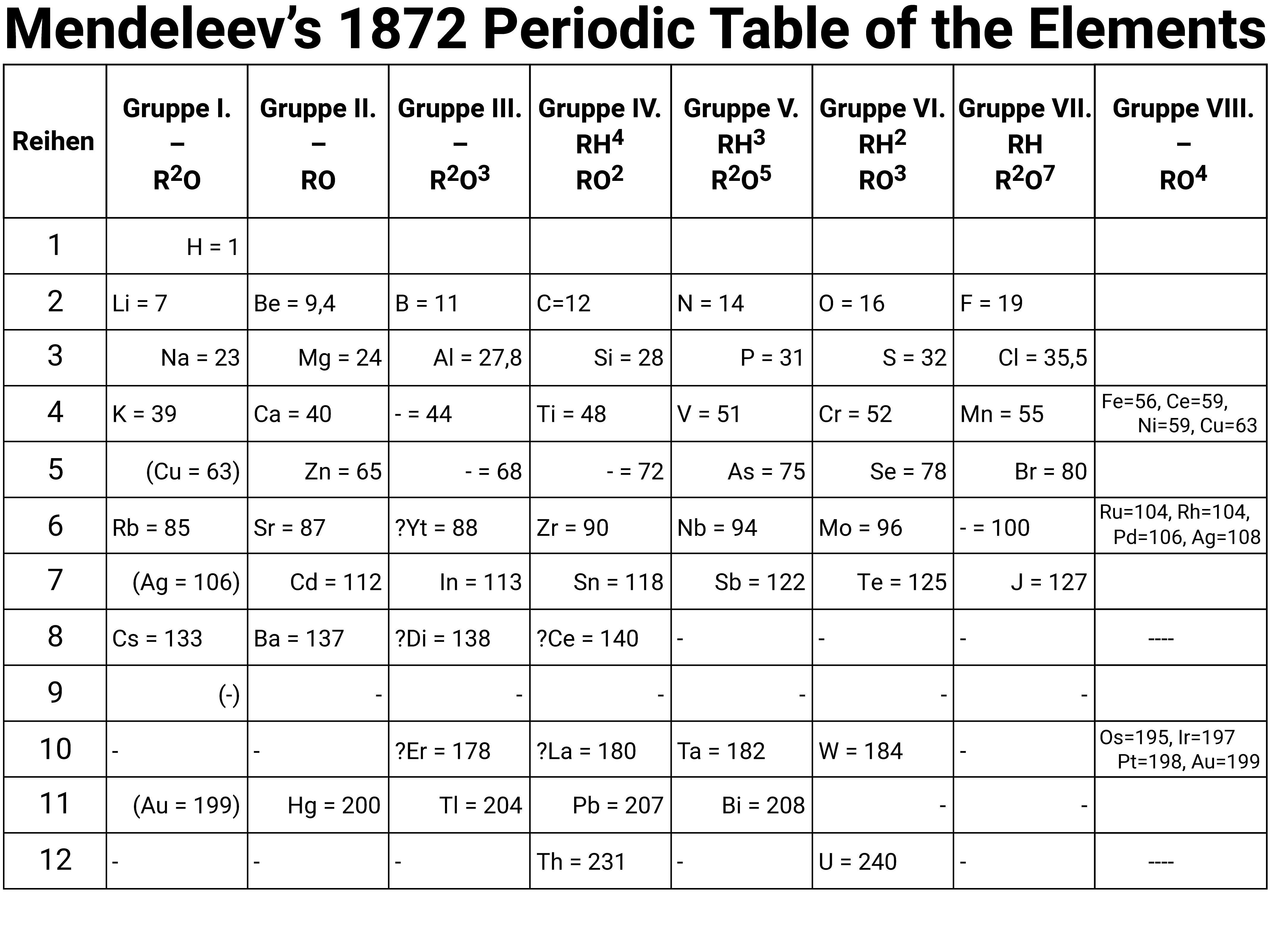
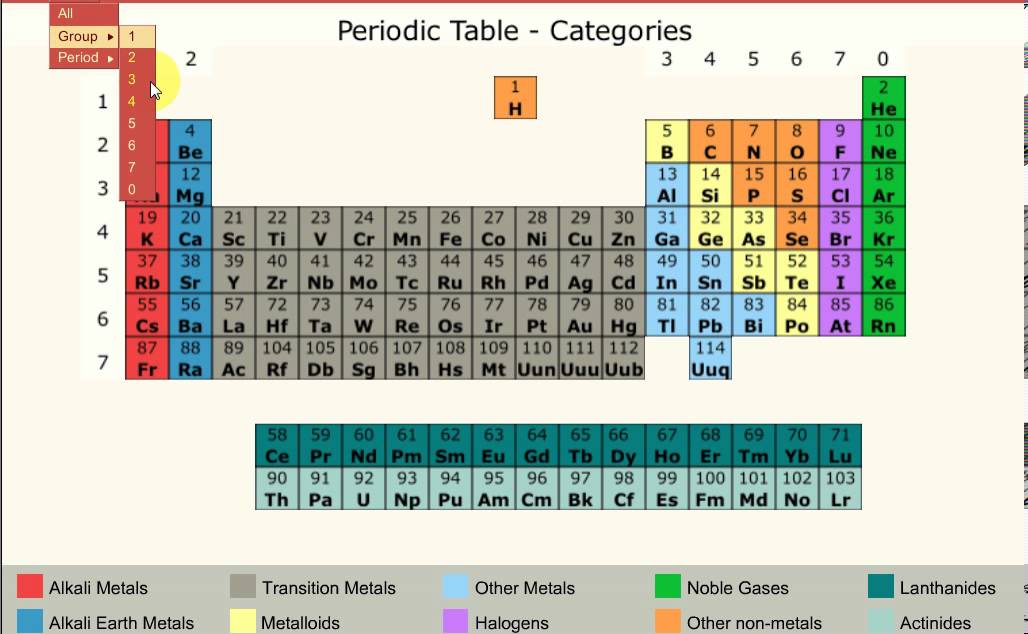
The Periodic Table In 1869 a Russian scientist called Dimitri Mendeleev published his Periodic Table which arranged the elements to show patterns in their chemical properties With just a few adjustments the modern Periodic Table was produced and became one of the most important tools in chemistryOr Having some additional hours of tuition with an experienced chemistry teacherThe elements in group 0 are called the noble gases They belong to the righthand column in the periodic table The noble gases are all chemically unreactive which means they are inert noble gases He helium, Ne neon, Ar argon, Kr krypton, Xe xenon, Rn radon;
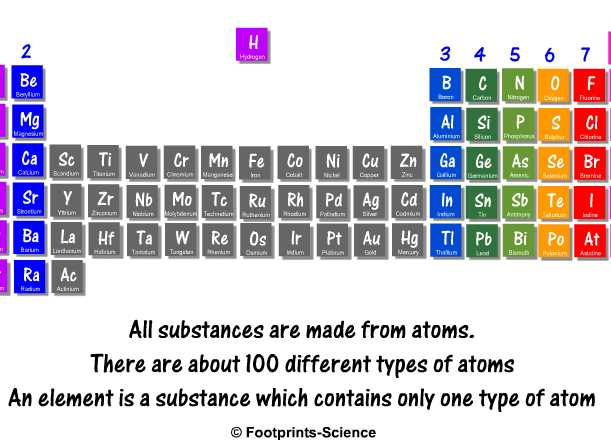



Periodic Table Animation Slide Footprints Science Gcse Science Animations And Quizzes



Igcse Gcse Chemistry Periodic Table Metals Group 1 9 2 Youtube
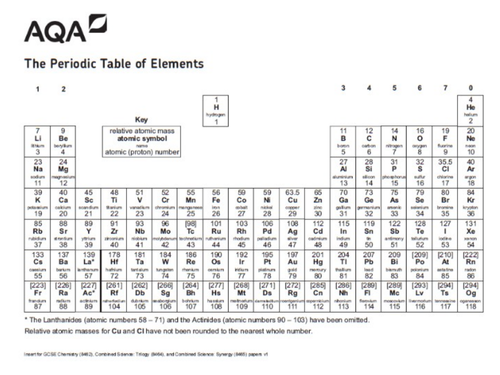
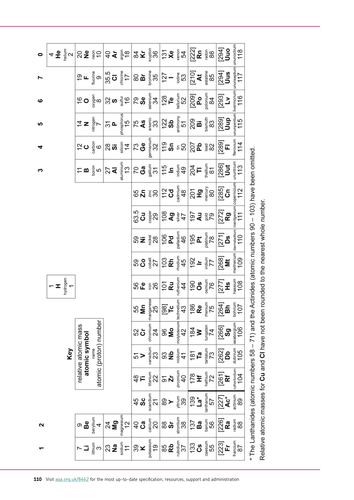
The Periodic Table of Elements 7 Li lithium 3 23 Na sodium 11 39 K potassium 19 85 Rb rubidium 37 133 Cs caesium 55 223 Fr 87 * The Lanthanides (atomic numbers 58 – 71) and the Actinides (atomic numbers 90 – 103) have been omitted Relative atomic masses forAtoms are the smallest part of elements that exist At present there are more than 100 different elements, all of which are shown on the periodic table Although more elements are discovered all the time and in 15 four new elements were added to the Periodic table (Tennessine, Nihonium, Moscovium and Oganesson)Element Properties Made by expert teachers




History Of The Periodic Table Gcse Chemistry Combined Science Aqa Revision Study Rocket




19 The Year Of The Periodic Table Rick Anderson
When it comes to preparing for your GCSE chemistry exam, this may mean Revising topics like the periodic table, atomic composition, or the fundamentals of atoms, elements, and compounds;GCSE Chemistry The Periodic Table Questions Includes The periodic table Development of the periodic table Explain why Mendeleevs table is useful in understanding new elements (2 marks) Title GCSE Chemistry, AQA, OCR, EDEXCEL The Development of the Periodic TableModel answers for Group &




Atomic Structure Knowledge Organiser Aqa Science Beyond
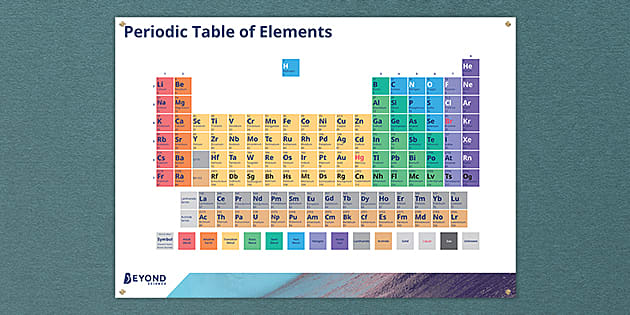



Periodic Table Of Elements Poster Ks3 Chemistry Beyond
There are two copies of the periodic table one in colourThe Modern Periodic Table What you need to know How the periodic table is now arranged Why is arrangement works What groups and periods represent The periodic table is a table of all of the known atoms in existence, and it looks like this There is usually a key at the top of the table, which tells you what all the information means inPeriodic Table The Royal Society of Chemistry's interactive periodic table features history, alchemy, podcasts, videos, and data trends across the periodic table Click the tabs at the top to explore each section Use the buttons above to change your view of the periodic table and view Murray Robertson's stunning Visual Elements artwork




Gcse Periodic Table Revise The Order And Types Of Elements




Periodic Trends Cie Igcse Chemistry Revision Notes
Group Code Registration Form;GCSE Chemistry – Periodic table Last updated Private Eduqas GCSE Science – Chemistry foundationNEW 16 GCSE Chemistry The Periodic Table full lesson Lesson Resource Pack, contains Brand new Powerpoint lesson Blank periodic table to colour in Diagram of an element to label A worksheet about subatomic particles A homework sheet from past exam questions relating to periodic table If this resource is popular I will upload more




Gcse Chemistry Revision Checklist For Separates And Trilogy By Ullswater Community College Issuu



Http Www Wvacademy Org Wp Content Uploads Sites 7 01 Chem 1 Atomic Structure And The Periodic Table 1 Pdf
Periodic Table Group 1 Elements A knowledge of the periodic table is a vital part of GCSE Chemistry In this quiz we look at the group 1 elements the alkali metals After many years of work by many different scientists, the periodic table was devised The scientist given most credit was a Russian, Dimitri Mendeleev (pronounced Mendellayef)Describe how Mendeleev arranged the elements known at that time, in a periodic table by using properties of these elements and their compounds In 1869 Dmitri Mendeleev created his first draft of the Periodic Table He started by writing down the names of the 50 elements known at that time on pieces of paper and placing them on a boardThe periodic table is classified so that all elements are arranged in a series of rows and columns in accordance to the element's chemical properties




Periodic Table Of The Elements In Continental English Language Michael Canov From Czech Republic




Printable Periodic Tables Pdf In 21 Periodic Table How To Memorize Things Problem Solving Worksheet
O Level KS4 science CHEMISTRY Revision Notes with links to more detailed GCSE notes advanced A level chemistry notes Subi ndex of contents of this page 1Chemistry GCSE Revision Atomic Structure And The Periodic Table Jan th January 21 Lucy BellYoung T By Lucy BellYoung Among the fundamental concepts in GCSE chemistry that you should revise is the atomic structure and the arrangement of elements in the periodic tableThe Periodic Table was developed in 1869 and it revolutionised science It's organised into groups and periods, and an element's position can tell us a great deal To revise what you learned in Year 10 and year 11, have a go at this GCSE chemistry quiz and get your feet under the periodic Table!




Atomic Structure The Periodic Table Cie Igcse Chemistry Revision Notes



Ks4 Gcse Chemistry The Periodic Table Revision Resources For Dyslexics
GCSE (Level 2) Further Maths;On the Periodic Table you will find every element that we know about, which in turn is a list of every atom that we have discovered It is ordered by atomic number, meaning that reading from left to right increases the proton number by one every time The Periodic Table is arranged in such a way that elements with similar properties can be found together in columns called 'groups' forElements of the Periodic Table A notebook containing some interesting facts about the periodic table Perfect gift for scientists and budding scientists!




Ks4 Gcse Chemistry The Periodic Table Revision Resources For Dyslexics
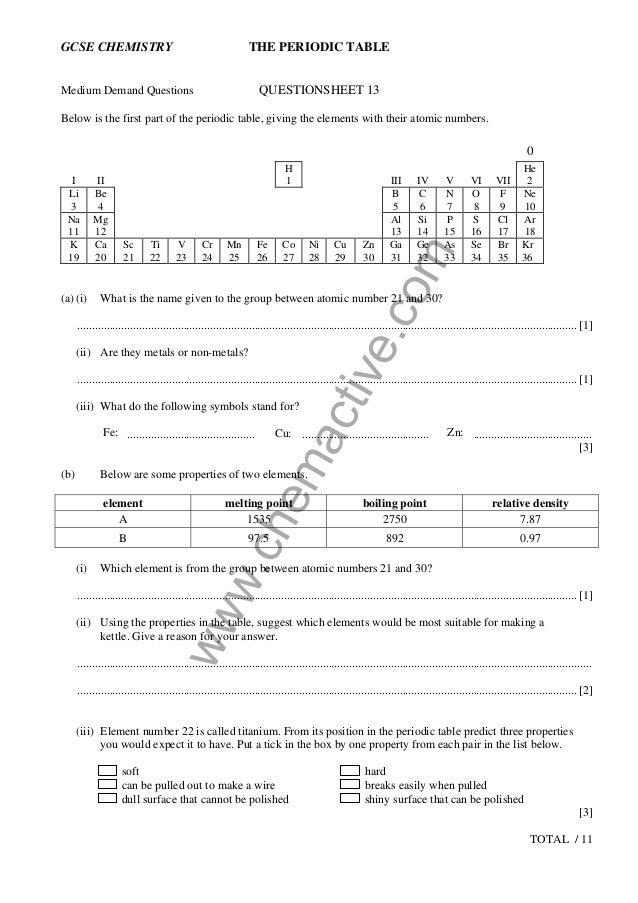



Periodic Questions
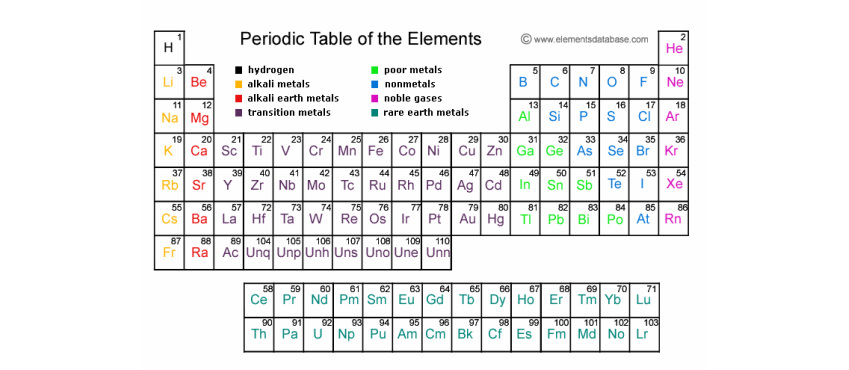
Most elements are metals, rather than nonmetals Each element has its own chemical symbol, made from letters Only elements are found in the periodic table,Atoms, Elements and Compounds All substances are made of atoms An atom is the smallest part of an element that can exist Atoms of each element are represented by a chemical symbol, for example O represents an atom of oxygen Na represents an atom of sodium There are about 100 different elements Elements are shown in the Periodic TableElements with atomic numbers 112 – 116 have been reported but not fully authenticated lead N 14 7 nitrogen P 31 15 phosphorus As 75 33 arsenic Sb 122 51 antimony Bi 9 bismuth O 16 8 oxygen S 32 16 sulfur Se 79 34 selenium Te 128 52 tellurium Po 9 84 polonium F 19 9 fluorine Cl 355 17 chlorine Br 80 35 bromine I 127 53 iodine At 210 85 astatine Ne 10 neon He 4 2



3



Swot Revision
Going through past exam papers from your exam board;The atomic structure and the periodic table resource is the first lesson in the GCSE Atomic Structure and the Periodic Table unit The lesson pack covers atoms, elements and isotopes The pack includes a PowerPoint, activities, examstyle questions and teaching ideas Everything you need to kickstart this interesting topic!The noble gases have the following properties in common




Revise Gcse Chemistry Revision Podcast Podtail




The Periodic Table Chemistry Gcse Revision
CIE IGCSE Chemistry exam revision with multiple choice questions &The periodic table is a classification of all known elements >1 10 of 3,286 search results for 'periodic table' GCSE Combined Science Trilogy Higher Questions Chemistry Test 1 Atomic structure and the periodic table and Bonding, structure and the properties of matter 21 (1319k) Chemistry Test 1 Atomic structure and the periodic table and Bonding, structure and the properties of matter (Higher)




Periodic Table Elements Wild Country Fine Arts




Aqa Gcse Chemistry Teacher Pack By Collins Issuu
So if an element has 3 valence electrons, it will be in group 3 And if it has 4 occupied energy shells, it will be in period 4 We have two types of elements in the periodic table These are Metals and Nonmetals As we move in the periodic table from the left to the right, the metallic properties of elements decrease Metals include MagnesiumThe group number of an element which is given on the periodic table indicates the number of electrons in the outer shell (valence electrons) This rule holds true for all elements except helium;The Periodic Table All the elements of the periodic table are arranged in order of increasing atomic (proton) number The table was named the periodic table because similar properties occur at regular intervals Elements with similar properties are in columns called groups Elements to the left of this line are metals




1 01 Elements And Compounds Sjp1618gcsechem



Http Hannahhelpchemistry Blogspot Com 13 02 113 Understand That Periodic Table Is Html
Although is in group 0, it has only one shell, the first and innermost shell, which holds only 2 electrons;He wrote chemistry books and was looking for ways to organise the known elements He published his first periodic table of the elements in 1869 In it, he arranged the elementsThe Periodic Table shows Metals (in brown) and NonMetals (in blue) Transition Metals (in pink) have no group number See How to use the Periodic Table or click on one of the elements above, or visit chemical symbols Printable Periodic Table
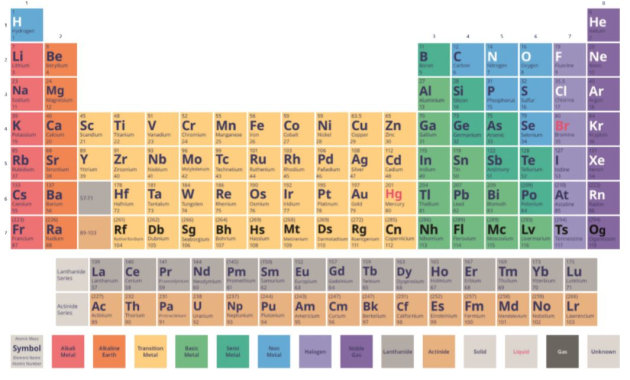



What Is The Periodic Table Answered Twinkl Teaching Wiki




The Periodic Table Aqa Gcse Chemistry Questions Answers
Summer Start for ALevel;In 1869, a Russian chemist named Dmitri Mendeléev published a periodic table He arranged the elements known at the time in order of increasing relative atomic mass and showed that elements of similar properties reoccurred at regular intervals The table he produced had elements with similar properties fall into the same vertical columnThe EPQ – Ultimate Guide;
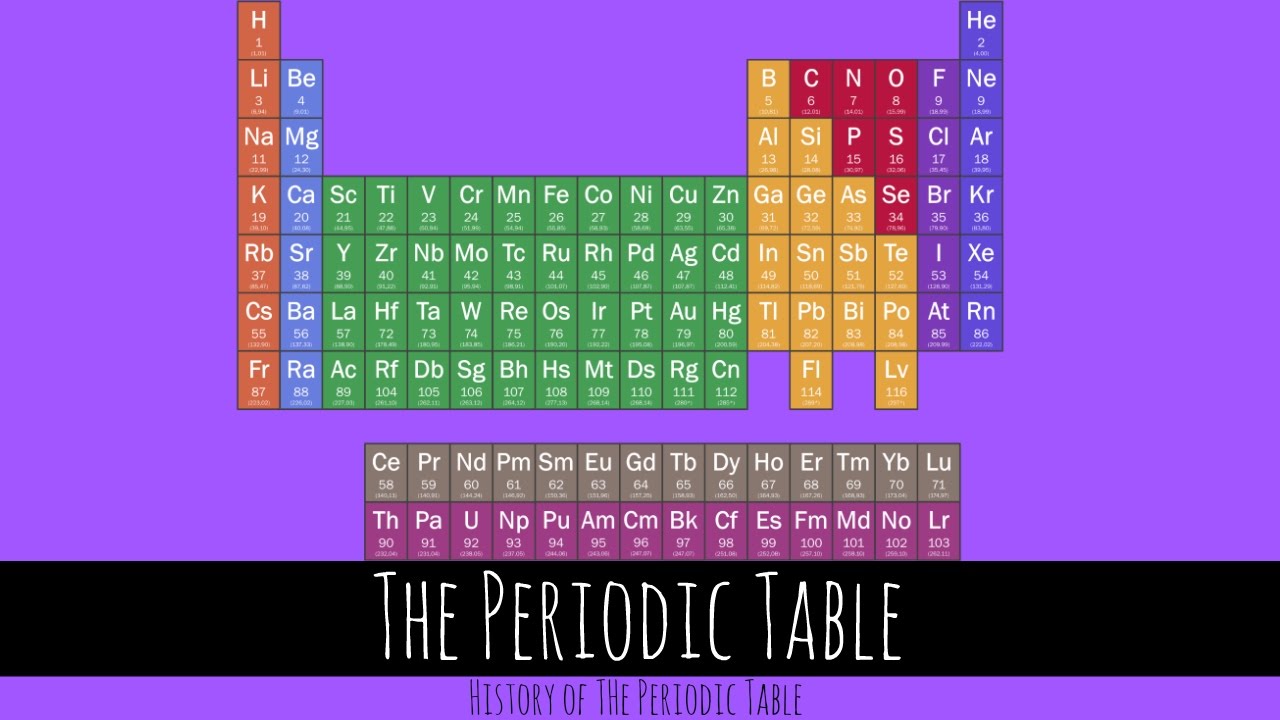



The Periodic Table History Of The Periodic Table Gcse Chemistry Youtube



Pass My Exams Easy Exam Revision Notes For Gsce Chemistry
The PERIODIC TABLE of elements An INTRODUCTION and OVERVIEW of the PERIODIC TABLE (both modern and historic periodic tables) Doc Brown's 91 GCSE/IGCSE &We can use the group number to predict how elements will react as the number ofThe Periodic Table >




Buy Stickers Magic Periodic Table Poster Display With Elements Home School Science Educational Wall Chart Wall Sticker Ks3 Ks4 Gcse Chemistry Student Teacher Usv 024 Online In Uk B08b4xrg7c



Gcse Creative Chemistry
Home / GCSE Physics / Elements of the Periodic Table A notebook containing some interesting facts about the periodic tableThe GCSE Chemistry periodic table of elements is something which you have to get to know pretty well for your exam From Mendeleev and the history of the periodic table to the alkali metals and the halogens, the periodic table contains many subtopics within it, each of which is very important for the Edexcel, OCR and AQA GCSE Chemistry examsThis quiz is designed to test your understanding of the periodic table something which is a must for GCSE Chemistry students In it you will need to show your knowledge of the elements' reactions, symbols, physical and chemical properties, the ions they form, their place in the periodic table and their electron structure, as well as periodic table patterns




History Of The Periodic Table Aqa Gcse Chemistry Revision Notes




Ks4 The Periodic Table Teachit Science




Periodic Table Questions Gcse Curriculum Press




Exam Style Questions S Cool The Revision Website




Gcse Periodic Table Introduction Worksheet Questions On Basic Ideas Of Its Structure Igcse Ks4 Science Revision Questions




Arranging The Elements Aqa Gcse Combined Chemistry Revision Notes




History Of The Periodic Table Gcse Chemistry Combined Science Aqa Revision Study Rocket



Pass My Exams Easy Exam Revision Notes For Gsce Chemistry




Ks4 Aqa Gcse Chemistry Science Atomic Structure Periodic Table Revision Knowledge Organiser Teaching Resources




Aqa 9 1 Gcse Chemistry Unit 1 Complete Teaching Bundle Teaching Resources




Gcse Practice Exam Question Worksheet On The Historical Development Of The Periodic Table Igcse Ks4 Science Revision Questions
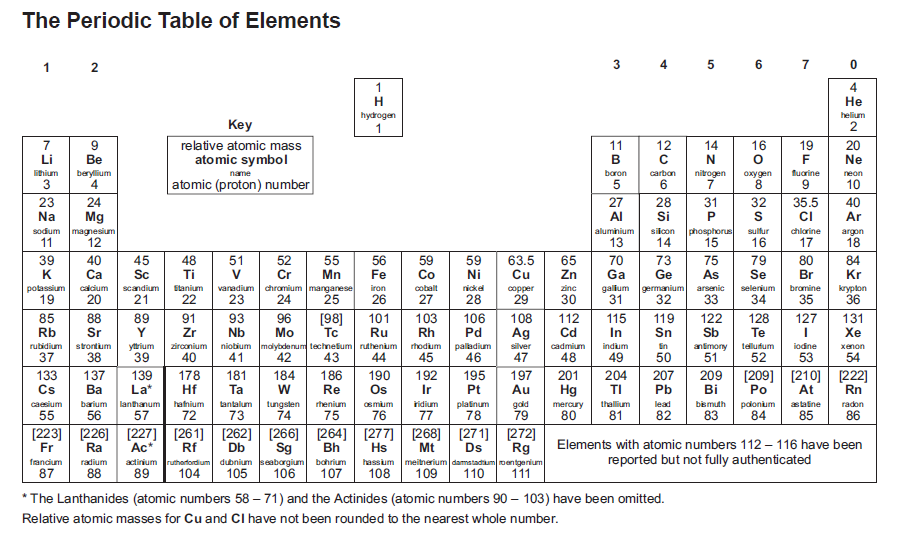



19 The Year Of The Periodic Table Rick Anderson
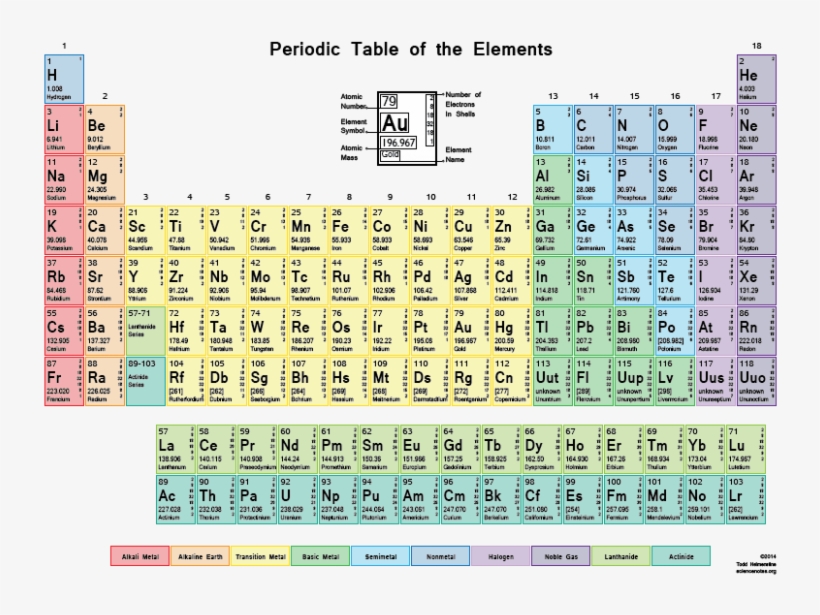



Color Periodic Table With Shells Gcse Periodic Table With Mass And Atomic Numbers Free Transparent Png Download Pngkey



Atomic Structure Revision Notes In Gcse Chemistry
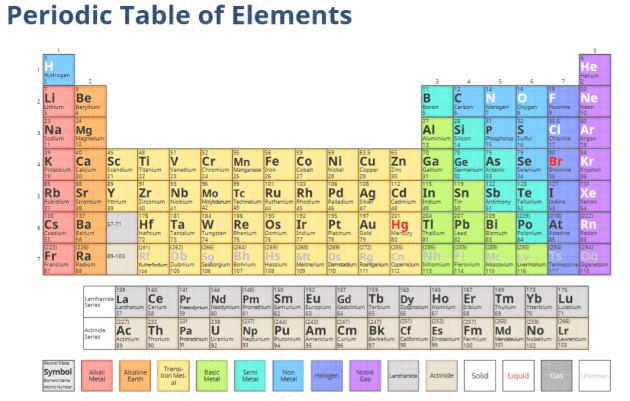



What Is An Element Answered Twinkl Teaching Wiki




Aqa Gcse Chemistry C2 Periodic Table Worksheets Teaching Resources



Periodic Table Of The Elements Gcse Chemistry Revision Centre




Gcse Chemistry The Periodic Table Aqa 9 1 Youtube




Stickers Magic Periodic Table Poster Display With Elements Home School Science Educational Wall Chart Wall Sticker Ks3 Ks4 Gcse Chemistry Student Teacher Usv 024 Amazon Co Uk Stationery Office Supplies




Free Printable Periodic Tables Pdf And Png Science Notes And Projects



The Periodic Table Gcse The Science Hive




Gcse Periodic Table Revise The Elements In Group One



The Periodic Table Aqa C1 Revisechemistry Uk



Aqa Gcse Chemistry Trilogy Paper 1 Revision Calculations Teaching Resources




Placing Elements In Order S Cool The Revision Website



Gcse Chemistry The Periodic Table Aqa 9 1 Youtube




Stickers Magic Periodic Table Poster Display With Elements Home School Science Educational Wall Chart Wall Sticker Ks3 Ks4 Gcse Chemistry Student Teacher Usv 024 Amazon Co Uk Stationery Office Supplies



1




Aqa Science Chemistry Periodic Table Poster 1 2m Wide And 0 85m Tall Teaching Resources




Buy Stickers Magic Periodic Table Poster Display With Elements Home School Science Educational Wall Chart Wall Sticker Ks3 Ks4 Gcse Chemistry Student Teacher Usv 024 Online In Uk B08b4xrg7c



Gcse Chemistry The Periodic Table Links To All Of The Elements Gcse Science



What Is The Periodic Table Definition From Seneca Learning




C1 1 The Periodic Table Secondary Science 4 All



The Periodic Table Igcse Chemistry Revision Help




Edexcel Igcse Chemistry The Periodic Table Expert Guidance By Mahima Laroyia




Edexcel Igcse Chemistry The Periodic Table Expert Guidance By Mahima Laroyia




Gcse Chemistry Atomic Structure And The Periodic Table Diagram Quizlet




Chemistry Students What Is Your Favourite Element From The Periodic Table And Why The Student Room




Periodic Table Groups Periods Trends Patterns Comparison Properties Of Metals Non Metals Allotropes Summary Overview Gcse Chemistry Revison Notes Igcse O Level Ks4 Science




c Gcse Bitesize Groups Gcse Chemistry Periodic Table Chemistry Revision




C1 1 Fundamental Ideas In Chemistry The Periodic Table Cgp Books Gcse Science Chemistry Lessons Chemistry Education




Edexcel Gcse Chemistry Topic 1 The Periodic Table Diagram Quizlet



An Introduction To The Periodic Table New 16 Gcse Chemistry Teaching Resources



Aqa Gcse Chemistry Unit 1 Fundamental Ideas Pt2 Periodic Table Youtube




What Is An Element Definition And Examples What Is An Element Periodic Table Element Chemistry
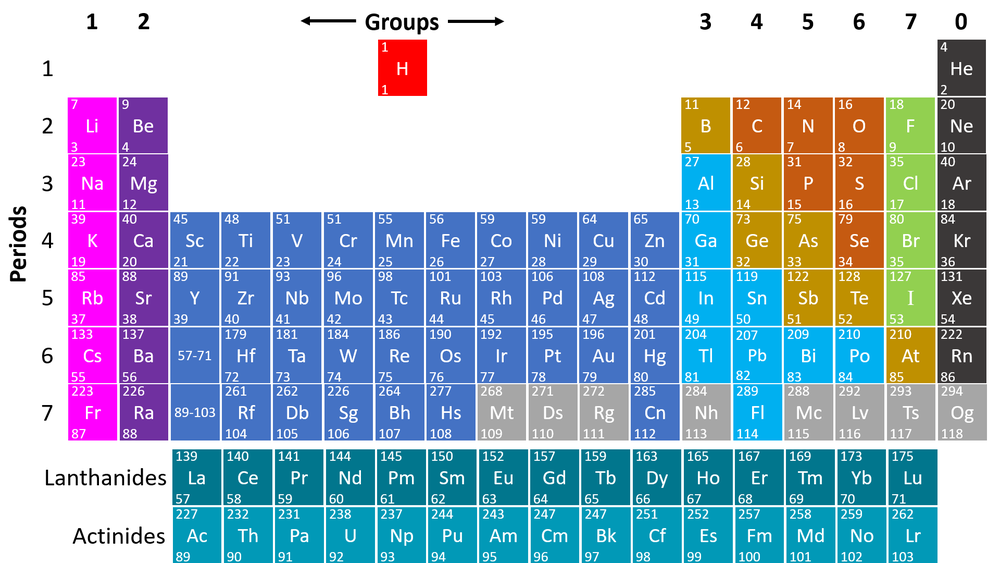



Periodic Table Key Stage Wiki




Transition Elements Cie Igcse Chemistry Revision Notes




Periodic Table Groups Periods Trends Patterns Comparison Properties Of Metals Non Metals Allotropes Summary Overview Gcse Chemistry Revison Notes Igcse O Level Ks4 Science




Free Printable Periodic Tables Pdf And Png Science Notes And Projects



Pass My Exams Easy Exam Revision Notes For Gsce Chemistry



Www Rewardinglearning Org Uk Common Includes Microsite Doc Link Aspx Docid 1
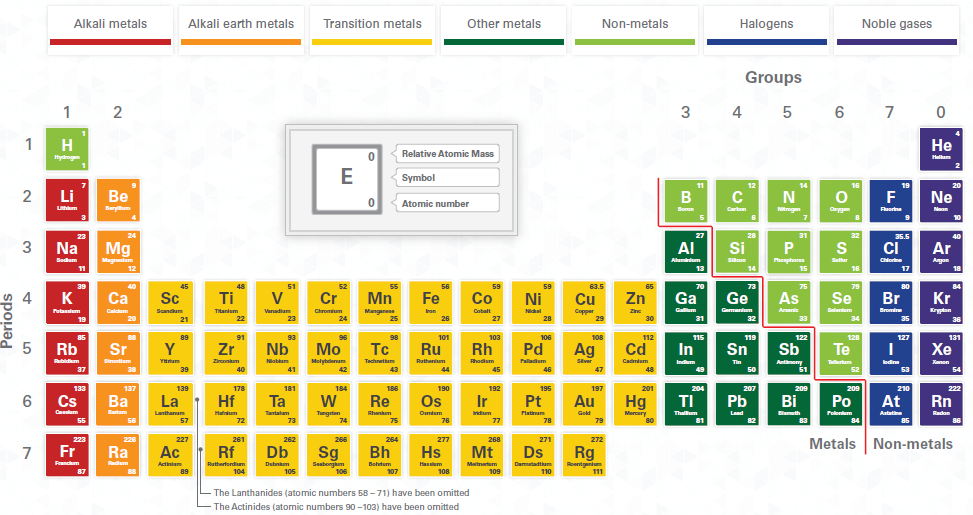



19 The Year Of The Periodic Table Rick Anderson




Swanshurst School Transition Pack For A Level Chemistry




Stickers Magic Periodic Table Poster Wall Sticker With Elements Home School Science Educational Wall Chart Ks3 Ks4 Gcse Chemistry Student Teacher Usv 023 Amazon Co Uk Stationery Office Supplies




Gcse Periodic Table As Credited To Dmitri Mendeleev Periodic Table Gcse Chemistry Dmitri Mendeleev



Aqa Gcse Chemistry 11 Doc 244 Kb



Ks4 Chemistry The Periodic Table Ppt Download




03 The Periodic Table Hallman Chemistry Unit



1



Http Www Lordwilliams Oxon Sch Uk Force Download Cfm Id 4859
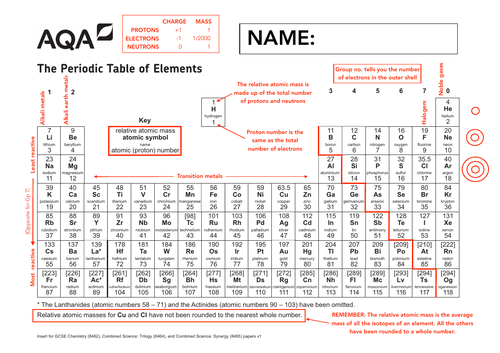



Annotated Periodic Table Aqa Chemistry Teaching Resources



3



Filestore Aqa Org Uk Resources Science Aqa 8462 8464 8465 Ins Pt Pdf




New 9 1 Aqa Gcse Chemistry Paper 1 The Periodic Table Complete Revision Summary Expert Guidance By Mahima Laroyia



Www Ocr Org Uk Images Periodic Table Of The Elements Poster Pdf
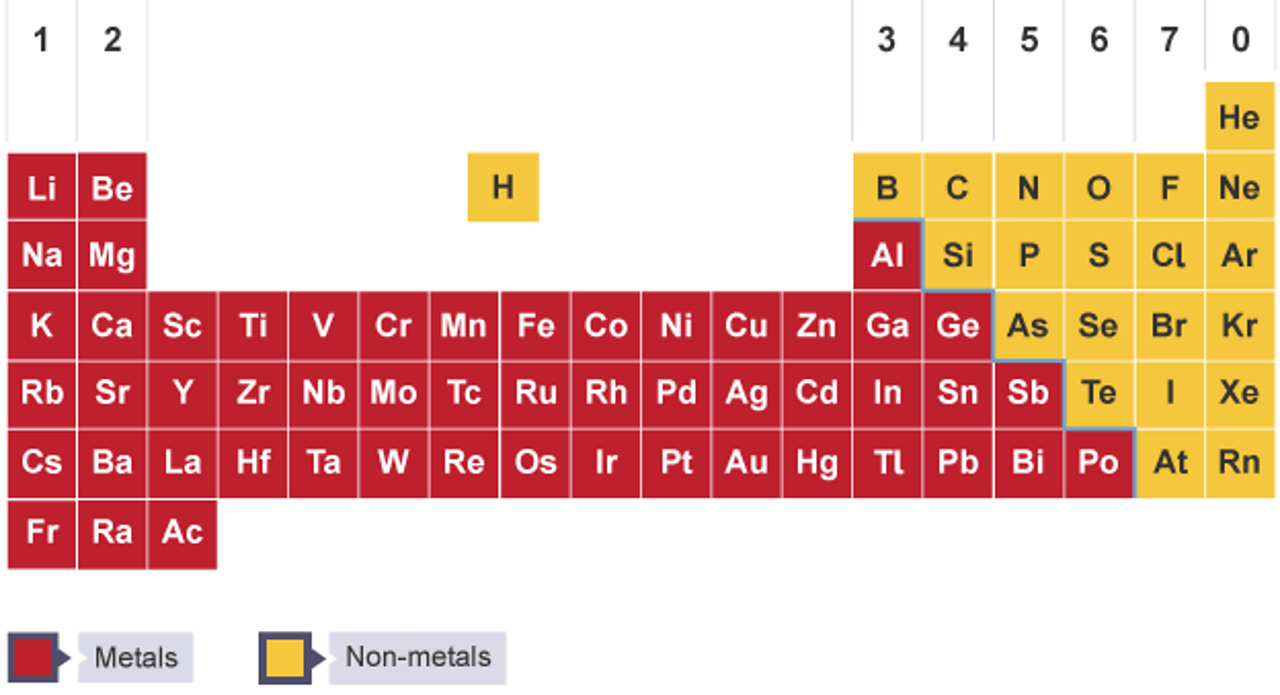



The Periodic Table The Periodic Table Ks3 Chemistry Revision c Bitesize




Aqa Gcse 9 1 Chemistry Quiz On Topic 1 Atomic Structure And The Periodic Table



Chemistry Aqa Further Gcse Revision Cards In Gcse Chemistry




World S Snazziest Periodic Table Card Print With Free Mini Periodic Table Ebay



Pass My Exams Easy Exam Revision Notes For Gsce Chemistry


